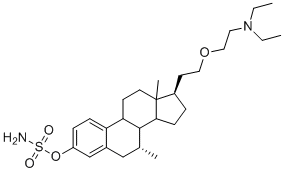
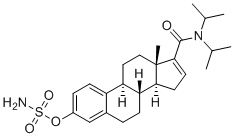
Steroid Sulfatase (STS)
STS (EC 3.1.6.2, aryl sulfatase C) is the enzyme responsible for the hydrolysis of alkyl [e.g., dehydroepiandrosterone (DHEA) sulfate (DHEAS)] and aryl steroid sulfates to their unconjugated forms. STS is a member of a superfamily of 12 different mammalian sulfatases. The gene consists of 10 exons and spans 146 kb, with the intron sizes ranging from 102 bp up to 35 kb. It encodes a protein of 583 amino acids, with a signal peptide of 21–23 peptides and four potential glycosylation sites of which at least two are used, at asparagine residues 47 and 259. Inactivation of the STS gene results in X-linked ichthyosis (X-LI), one of the most prevalent human inborn errors of metabolism. All the point mutations reported are located in the carboxyl region of the STS enzyme, which is thought to be important for substrate binding.
The cytokines TNF and IL-6 both up-regulate STS enzyme activity in MCF-7 breast cancer cells. However, upon further investigation, this up-regulation appeared to be posttranslationally mediated rather than occurring via any changes in gene transcription or mRNA stability. In contrast, the inflammatory cytokine IL-1 decreases the activity and expression of STS mRNA in human endometrial stromal cells, in a dose-dependent manner, and this effect is antagonized by the IL-1 receptor antagonist. IL-1 also suppressed STS activity and mRNA expression in vascular smooth muscle cells derived from human aortas. Both basic fibroblast growth factor and IGF-I were found to increase STS activity in a dose- and time-dependent manner in MCF-7 and MDAMB-231 breast cancer cells.1.Reed MJ,et al. Endocr Rev. 2005;26(2):171–202.
The cytokines TNF and IL-6 both up-regulate STS enzyme activity in MCF-7 breast cancer cells. However, upon further investigation, this up-regulation appeared to be posttranslationally mediated rather than occurring via any changes in gene transcription or mRNA stability. In contrast, the inflammatory cytokine IL-1 decreases the activity and expression of STS mRNA in human endometrial stromal cells, in a dose-dependent manner, and this effect is antagonized by the IL-1 receptor antagonist. IL-1 also suppressed STS activity and mRNA expression in vascular smooth muscle cells derived from human aortas. Both basic fibroblast growth factor and IGF-I were found to increase STS activity in a dose- and time-dependent manner in MCF-7 and MDAMB-231 breast cancer cells.1.Reed MJ,et al. Endocr Rev. 2005;26(2):171–202.
Metabolic Enzyme/Protease
11β-HSD(15)
15-PGDH(1)
ACC(10)
ACE(15)
AChE(47)
Adenylate Cyclase(12)
ALDH(14)
Aldose Reductase(5)
Aminopeptidase(19)
BACE(19)
Casein Kinase(51)
CAT(5)
Cathepsin(9)
CETP(13)
COMT(2)
CPG2(1)
CYPs(6)
Decarboxylase(3)
Dehydrogenase(131)
DGAT(4)
Dopamine beta-hydroxylase(2)
DPP(32)
Elastase(6)
FAAH(28)
Factor Xa(31)
Fatty Acid Synthase(17)
Ftase(2)
FXR(26)
Glucokinase(1)
GSNOR(2)
Guanylate Cyclase(13)
HMGCR(17)
IDH(7)
IDO(20)
IMPDH(2)
LDH(2)
LDL(8)
Lipase(17)
Lipid(12)
MAGL(6)
MAO(72)
MMP(78)
NAMPT(12)
Neprilysin(7)
Other Targets(10)
P450(112)
PAI-1(9)
Phosphatase(95)
Phospholipase(65)
PPAR(115)
Protein Phosphatase/PTP(6)
Renin(8)
Retinoid Receptor(37)
SCD(6)
Steroid Sulfatase (STS)(2)
Thioredoxin(1)
TPH(5)
Transferase(37)
Vitamin(44)
Xanthine Oxidase (XAO)(11)



 021-51111890
021-51111890 购物车(0)
购物车(0)
 sales@molnova.cn
sales@molnova.cn