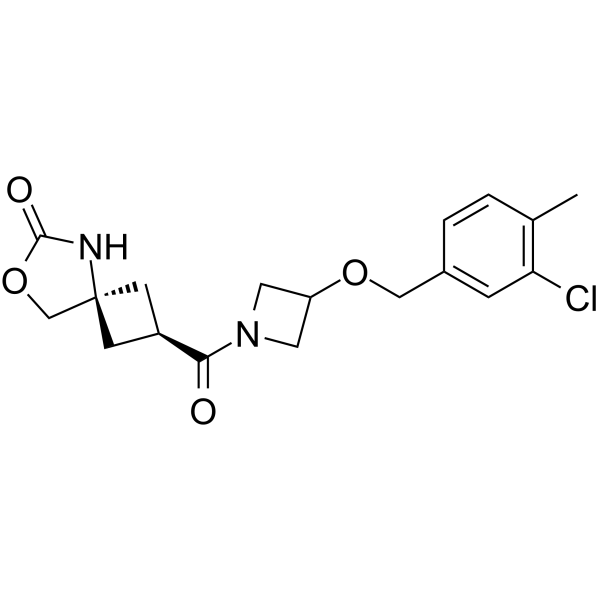
MAGL-IN-4
CAS No. 2135785-20-3
MAGL-IN-4 ( His121 ARG57 )
产品货号. M28832 CAS No. 2135785-20-3
MAGL-IN-4 是一种单酰基甘油脂肪酶 (MAGL) 抑制剂,用于治疗中枢神经系统相关疾病。
纯度: >98% (HPLC)
 COA
COA
 Datasheet
Datasheet
 HNMR
HNMR
 HPLC
HPLC
 MSDS
MSDS
 Handing Instructions
Handing Instructions
| 规格 | 价格/人民币 | 库存 | 数量 |
| 5MG | ¥3208 | 有现货 |


|
| 10MG | ¥4771 | 有现货 |


|
| 25MG | ¥7655 | 有现货 |


|
| 50MG | ¥10368 | 有现货 |


|
| 100MG | ¥14013 | 有现货 |


|
| 500MG | ¥28026 | 有现货 |


|
| 1G | 获取报价 | 有现货 |


|
生物学信息
-
产品名称MAGL-IN-4
-
注意事项本公司产品仅用于科研实验,不得用于人体或动物的临床与诊断
-
产品简述MAGL-IN-4 是一种单酰基甘油脂肪酶 (MAGL) 抑制剂,用于治疗中枢神经系统相关疾病。
-
产品描述MAGL-IN-4 is one of the monoacylglycerol lipase (MAGL) inhibitors in central nervous system-related diseases.(In Vitro):MAGL-IN-4 showed potent in vitro MAGL inhibitory activity (IC50 6.2 nM), good oral absorption and blood-brain barrier penetration.(In Vivo):MAGL-IN-4 showed significant pharmacodynamic changes (2-arachidonoylglycerol increase and arachidonic acid decrease) at 0.3-10 mg/kg, po. in mice.
-
体外实验MAGL-IN-4 (compound 4f) shows a high LLE (5.9), a logD of 2.3 for MAGL.MAGL-IN-4 exhibits no inhibition toward the closely related serine hydrolases (FAAH and ABHD6; all IC50>10000 nM). MAGL-IN-4 has no significant binding potentials to cannabinoid receptors (CB1: 19% and CB2: 5% at 10 μM), and low hERG liability (14.4% inh. at 10 μM, manual patch clamp, without BSA).
-
体内实验MAGL-IN-4 (compound 4f; 0.1-10 mg/kg; oral; single dose) results in a significant elevation in the level of 2-AG and reduction in that of arachidonic acid (AA) from 0.3 mg/kg in C57BL/6J mice.
-
同义词His121 ARG57
-
通路Metabolic Enzyme/Protease
-
靶点Lipase
-
受体——
-
研究领域——
-
适应症——
化学信息
-
CAS Number2135785-20-3
-
分子量364.82
-
分子式C18H21ClN2O4
-
纯度>98% (HPLC)
-
溶解度In Vitro:?DMSO : 100 mg/mL (274.11 mM)
-
SMILESO=C(OC1)N[C@@]21C[C@@H](C(N3CC(OCC4=CC=C(C)C(Cl)=C4)C3)=O)C2
-
化学全称——
运输与储存
-
储存条件(-20℃)
-
运输条件With Ice Pack
-
稳定性≥ 2 years
参考文献
1.Jatczak, K., et al. Preparation of selectively protected protoescigenin derivatives for synthesis of escin analogs and neoglycoconjugates. cent.eur.j.chem. 12, 1222–1231 (2014).



 021-51111890
021-51111890 购物车(0)
购物车(0)
 sales@molnova.cn
sales@molnova.cn











