Filaminast
CAS No. 141184-34-1
Filaminast ( WAY-PDA-641;PDA-641;WAY-123641 )
产品货号. M11714 CAS No. 141184-34-1
Filaminast (WAY-PDA-641;PDA-641;WAY-123641) is a potent and selective PDE4 inhibitor with IC50 of 420 nM, displays 36-fold selectivity over PDE3.
纯度: >98% (HPLC)
 COA
COA
 Datasheet
Datasheet
 HNMR
HNMR
 HPLC
HPLC
 MSDS
MSDS
 Handing Instructions
Handing Instructions
| 规格 | 价格/人民币 | 库存 | 数量 |
| 5MG | ¥7857 | 有现货 |


|
| 10MG | ¥10449 | 有现货 |


|
| 25MG | ¥15795 | 有现货 |


|
| 50MG | ¥21222 | 有现货 |


|
| 100MG | ¥28593 | 有现货 |


|
| 200MG | 获取报价 | 有现货 |


|
| 500MG | 获取报价 | 有现货 |


|
| 1G | 获取报价 | 有现货 |


|
生物学信息
-
产品名称Filaminast
-
注意事项本公司产品仅用于科研实验,不得用于人体或动物的临床与诊断
-
产品简述Filaminast (WAY-PDA-641;PDA-641;WAY-123641) is a potent and selective PDE4 inhibitor with IC50 of 420 nM, displays 36-fold selectivity over PDE3.
-
产品描述Filaminast (WAY-PDA-641;PDA-641;WAY-123641) is a potent and selective PDE4 inhibitor with IC50 of 420 nM, displays 36-fold selectivity over PDE3; significantly potentiates the relaxant effects of albuterol, reverses tracheal contractions induced by prostaglandin F2 alpha, leukotriene D4 or histamine in a biphasic manner.Asthma Phase 2 Discontinued
-
同义词WAY-PDA-641;PDA-641;WAY-123641
-
通路Angiogenesis
-
靶点PDE
-
受体PDE
-
研究领域Inflammation/Immunology
-
适应症Asthma
化学信息
-
CAS Number141184-34-1
-
分子量292.34
-
分子式C15H20N2O4
-
纯度>98% (HPLC)
-
溶解度——
-
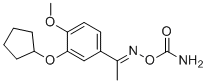
SMILESC(\C)(=N/OC(=O)N)c1cc(c(cc1)OC)OC1CCCC1
-
化学全称1-(3-(Cyclopentyloxy)-4-methoxyphenyl)ethanone-(E)-O-(aminocarbonyl)oxime
运输与储存
-
储存条件(-20℃)
-
运输条件With Ice Pack
-
稳定性≥ 2 years
参考文献
1. Heaslip RJ, et al. J Pharmacol Exp Ther. 1994 Feb;268(2):888-96.
产品手册



关联产品
-
NSP-805
NSP-805 is a potent and selective guinea pig cardiac phosphodiesterase 3 (PDE3) inhibitor.
-
Anagrelide
Anagrelide is a Platelet-reducing Agent. The mechanism of action of anagrelide is as a Phosphodiesterase 3 Inhibitor.
-
Mirodenafil
Mirodenafil is a PDE-5 inhibitor developed for the treatment of erectile dysfunction.The pharmacoki-netics of mirodenafil were not significantly altered by this concurrent administration. Mirodenafil (50 or 100 mg), obviously improved erectile function and was well tolerated in a representative population of Korean men with broad-spectrum ED of various etiologies and severities.



 021-51111890
021-51111890 购物车()
购物车()
 sales@molnova.cn
sales@molnova.cn







