Cadazolid
CAS No. 1025097-10-2
Cadazolid ( ACT-179811 | Cadazolid. )
产品货号. M17120 CAS No. 1025097-10-2
Cadazolid 是一种恶唑烷酮型抗生素,具有对抗革兰氏阳性菌(包括艰难梭菌)的活性。
纯度: >98% (HPLC)
 COA
COA
 Datasheet
Datasheet
 HNMR
HNMR
 HPLC
HPLC
 MSDS
MSDS
 Handing Instructions
Handing Instructions
| 规格 | 价格/人民币 | 库存 | 数量 |
| 2MG | ¥356 | 有现货 |


|
| 5MG | ¥583 | 有现货 |


|
| 10MG | ¥988 | 有现货 |


|
| 25MG | ¥1798 | 有现货 |


|
| 50MG | ¥2989 | 有现货 |


|
| 100MG | ¥4447 | 有现货 |


|
| 200MG | 获取报价 | 有现货 |


|
| 500MG | 获取报价 | 有现货 |


|
| 1G | 获取报价 | 有现货 |


|
生物学信息
-
产品名称Cadazolid
-
注意事项本公司产品仅用于科研实验,不得用于人体或动物的临床与诊断
-
产品简述Cadazolid 是一种恶唑烷酮型抗生素,具有对抗革兰氏阳性菌(包括艰难梭菌)的活性。
-
产品描述Cadazolid, also known ACT-179811, is a novel fluoroquinolone-oxazolidinone antibiotic and a protein synthesis inhibitor. Cadazolid may be potentially useful for the treatment of Clostridium difficile infection. Cadazolid exhibits potent in vitro activity against Clostridium difficile, including the epidemic BI/NAP1/027 strain. Clostridium difficile infection (CDI), the main cause of nosocomial infectious diarrhea, results from the growth of toxin-producing C. difficile in the colon following disruption of the normal enteric microbiota, usually as a consequence of antibiotic therapy.(In Vitro):Cadazolid is a new antibiotic in development for the treatment of Clostridium difficile-associated diarrhea. Cadazolid is active against all (including linezolid- and moxifloxacin-resistant) Clostridium difficile strains (MIC90 0.125, range 0.03-0.25 mg/L). The cadazolid geometric mean MIC is 152-fold, 16-fold, 9-fold and 7-fold lower than those of moxifloxacin, linezolid, metronidazole and vancomycin, respectively. Both cadazolid dosing regimens rapidly reduce Clostridium difficile viable counts and cytotoxin with no evidence of recurrence. Cadazolid levels persists at 50-100-fold supra-MIC for 14 days post-dosing. Cadazolid inhibition of enumerated gut microflora is limited, with the exception of bifidobacteria; Bacteroides fragilis group and Lactobacillus spp. counts are unaffected. There is no evidence for selection of strains resistant to cadazolid, quinolones or linezolid. (In Vivo):Cadazolid is well tolerated up to 3000 mg given twice daily for 10 days. The most common adverse event is headache, with no observed relationship between dose or treatment duration and adverse events. Plasma concentrations of cadazolid are low. No plasma concentrations >3.3 ng/mL are observed after single doses or >6.9 ng/mL after 10 days of multiple doses. Food increased the mean Cmax from 0.73 to 1.87 ng/mL and mean AUC0–t from 3.13 to 15.69 ng·h/mL after a single 300 mg dose. The increase in systemic exposure to cadazolid across doses is less than dose-proportional. The mean cumulative faecal recovery is 81.0%–93.5%. Urinary recovery of unchanged compound is less than 0.015%.
-
体外实验Cadazolid is a new antibiotic in development for the treatment of Clostridium difficile-associated diarrhea. Cadazolid is active against all (including linezolid- and moxifloxacin-resistant) Clostridium difficile strains (MIC90 0.125, range 0.03-0.25 mg/L). The cadazolid geometric mean MIC is 152-fold, 16-fold, 9-fold and 7-fold lower than those of moxifloxacin, linezolid, metronidazole and vancomycin, respectively. Both cadazolid dosing regimens rapidly reduce Clostridium difficile viable counts and cytotoxin with no evidence of recurrence. Cadazolid levels persists at 50-100-fold supra-MIC for 14 days post-dosing. Cadazolid inhibition of enumerated gut microflora is limited, with the exception of bifidobacteria; Bacteroides fragilis group and Lactobacillus spp. counts are unaffected. There is no evidence for selection of strains resistant to cadazolid, quinolones or linezolid.
-
体内实验Cadazolid is well tolerated up to 3000 mg given twice daily for 10 days. The most common adverse event is headache, with no observed relationship between dose or treatment duration and adverse events. Plasma concentrations of cadazolid are low. No plasma concentrations >3.3 ng/mL are observed after single doses or >6.9 ng/mL after 10 days of multiple doses. Food increased the mean Cmax from 0.73 to 1.87 ng/mL and mean AUC0–t from 3.13 to 15.69 ng·h/mL after a single 300 mg dose. The increase in systemic exposure to cadazolid across doses is less than dose-proportional. The mean cumulative faecal recovery is 81.0%–93.5%. Urinary recovery of unchanged compound is less than 0.015%.
-
同义词ACT-179811 | Cadazolid.
-
通路Others
-
靶点Other Targets
-
受体Others
-
研究领域——
-
适应症——
化学信息
-
CAS Number1025097-10-2
-
分子量585.19
-
分子式C29H29F2N3O8
-
纯度>98% (HPLC)
-
溶解度DMSO : ≥ 150 mg/mL 256.17 mM
-
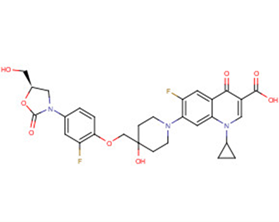
SMILESc1c(c(cc2c1n(cc(c2=O)C(=O)O)C1CC1)F)N1CCC(CC1)(COc1c(cc(cc1)N1C[C@@H](OC1=O)CO)F)O
-
化学全称1-Cyclopropyl-6-fluoro-7-[4-({2-fluoro-4-[(5R)-5-(hydroxymethyl)-2-oxo-1,3-oxazolidin-3-yl]phenoxy}methyl)-4-hydroxypiperidin-1-yl]-4-oxo-1,4-dihydroquinolin-3-carboxylic acid
运输与储存
-
储存条件(-20℃)
-
运输条件With Ice Pack
-
稳定性≥ 2 years
参考文献
1. Seiler P., et al. Cadazolid does not promote intestinal colonization of vancomycin-resistant enterococci in mice. Antimicrob Agents Chemother. 2015 Oct 26;60(1):628-31.
产品手册




关联产品
-
Phaclofen
Phaclofen 是一种选择性 GABAB receptor 拮抗剂。Phaclofen 是一种外周和中央的 baclofen 拮抗剂。酞氯芬在确定中枢和外周 GABA 和 (-)-baclofen 相互作用的 bicuculline-insensitive 受体的生理意义方面可能是一种潜在的化合物。
-
Trofinetide
Trofinetide (NNZ 2566 ) 是一种拟肽三肽 Gly-Pro-Glu,具有抗炎和神经保护特性,可预防缺氧缺血性脑损伤和神经变性。
-
Rhaponticin 6-O-gall...
Rhaponticin 6''-O-gallate showed inhibitory activity of NO production in lipopolysaccharide-activated macrophages, (IC50 = 11-69 microM).



 021-51111890
021-51111890 购物车()
购物车()
 sales@molnova.cn
sales@molnova.cn







