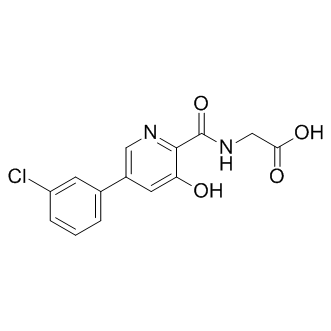
Vadadustat
CAS No. 1000025-07-9
Vadadustat ( PG-1016548 | PG1016548 | PG 1016548 | AKB-6548 )
产品货号. M10002 CAS No. 1000025-07-9
Vadadustat (PG-1016548, AKB-6548) 是一种新型、有效、口服活性的 HIF-PH 抑制剂,正在开发中,用于治疗非透析依赖性 (NDD) 和透析依赖性 CKD 患者的贫血。
纯度: >98% (HPLC)
 COA
COA
 Datasheet
Datasheet
 HNMR
HNMR
 HPLC
HPLC
 MSDS
MSDS
 Handing Instructions
Handing Instructions
| 规格 | 价格/人民币 | 库存 | 数量 |
| 2MG | ¥617 | 有现货 |


|
| 5MG | ¥1058 | 有现货 |


|
| 10MG | ¥1676 | 有现货 |


|
| 25MG | ¥4022 | 有现货 |


|
| 100MG | 获取报价 | 有现货 |


|
| 200MG | 获取报价 | 有现货 |


|
| 500MG | 获取报价 | 有现货 |


|
| 1G | 获取报价 | 有现货 |


|
生物学信息
-
产品名称Vadadustat
-
注意事项本公司产品仅用于科研实验,不得用于人体或动物的临床与诊断
-
产品简述Vadadustat (PG-1016548, AKB-6548) 是一种新型、有效、口服活性的 HIF-PH 抑制剂,正在开发中,用于治疗非透析依赖性 (NDD) 和透析依赖性 CKD 患者的贫血。
-
产品描述Vadadustat (PG-1016548, AKB-6548)?is a novel, potent, orally active HIF-PH inhibitor in development for the treatment of anemia in both nondialysis-dependent (NDD) and dialysis-dependent CKD; induces endogenous erythropoietin synthesis and enhances iron mobilization. Anemia Phase 3 Clinical.(In Vitro):Vadadustat induces endogenous erythropoietin synthesis and enhances iron mobilization. Vadadustat is well-tolerated in healthy volunteers and patients with chronic kidney disease, where it increases reticulocytes, plasma EPO, and Hb levels in a dose-dependent manner. The increase in plasma EPO levels seen with vadadustat is comparable in magnitude to that occurring physiologically at moderate altitude and shows a normal diurnal pattern with a return to baseline levels prior to the next dose. Vadadustat improves iron homeostasis by decreasing hepcidin and increasing transferrin levels. once-daily oral administration of vadadustat, titrated to increase and maintain Hb in the target range, may provide multiple advantages over conventional ESAs. Vadadustat is observed to have a half-life of approximately 4.5 hours. Overall, patients demonstrate an increase in Hb levels, from 9.91 g/dL at baseline to 10.54 g/dL by day 29. Ferritin levels decrease from 334.1 ng/mL at baseline to 271.7 ng/mL by day 29.
-
体外实验Vadadustat induces endogenous erythropoietin synthesis and enhances iron mobilization. Vadadustat is well-tolerated in healthy volunteers and patients with chronic kidney disease, where it increases reticulocytes, plasma EPO, and Hb levels in a dose-dependent manner. The increase in plasma EPO levels seen with vadadustat is comparable in magnitude to that occurring physiologically at moderate altitude and shows a normal diurnal pattern with a return to baseline levels prior to the next dose. Vadadustat improves iron homeostasis by decreasing hepcidin and increasing transferrin levels. once-daily oral administration of vadadustat, titrated to increase and maintain Hb in the target range, may provide multiple advantages over conventional ESAs. Vadadustat is observed to have a half-life of approximately 4.5 hours. Overall, patients demonstrate an increase in Hb levels, from 9.91 g/dL at baseline to 10.54 g/dL by day 29. Ferritin levels decrease from 334.1 ng/mL at baseline to 271.7 ng/mL by day 29.
-
体内实验——
-
同义词PG-1016548 | PG1016548 | PG 1016548 | AKB-6548
-
通路Angiogenesis
-
靶点HIF/HIF Prolyl-hydroxylase
-
受体HIF-PH
-
研究领域Other Indications
-
适应症Anemia
化学信息
-
CAS Number1000025-07-9
-
分子量306.70114
-
分子式C14H11ClN2O4
-
纯度>98% (HPLC)
-
溶解度DMSO: ≥ 33 mg/mL
-
SMILESO=C(O)CNC(C1=NC=C(C2=CC=CC(Cl)=C2)C=C1O)=O
-
化学全称Glycine, N-[[5-(3-chlorophenyl)-3-hydroxy-2-pyridinyl]carbonyl]-
运输与储存
-
储存条件(-20℃)
-
运输条件With Ice Pack
-
稳定性≥ 2 years
参考文献
1. Shalwitz R, et al. J Am Soc Nephrol. 2011;22:45A.
2. Pergola PE, et al. Kidney Int. 2016 Nov;90(5):1115-1122.
3. Martin ER, et al. Am J Nephrol. 2017;45(5):380-388.
产品手册




关联产品
-
HIF2α-IN-2
HIF2α-IN-2 是一种有效的、选择性的 HIF-2α PAS-B 结构域变构抑制剂,Kd 为 81 nM。
-
PT2385
PT2385 (PT-2385) 是一种有效的、选择性的、口服活性的 HIF2α 拮抗剂,与 HIF2α PAS-B 结构域 (Kd=50 nM) 结合并破坏 HIF2α/ARNT 二聚体的形成。
-
TAT-cyclo-CLLFVY
Selective HIF-1 dimerization inhibitor. Blocks protein-protein interaction of recombinant HIF-1α, but not HIF-2α, with HIF-1β (IC50 = 1.3 μM). Inhibits hypoxia-induced HIF-1 activity, and decreases VEGF and CAIX expression in osteosarcoma and breast cancer cells in vitro. Also reduces tubularization of hypoxic HUVECs.



 021-51111890
021-51111890 购物车(0)
购物车(0)
 sales@molnova.cn
sales@molnova.cn







