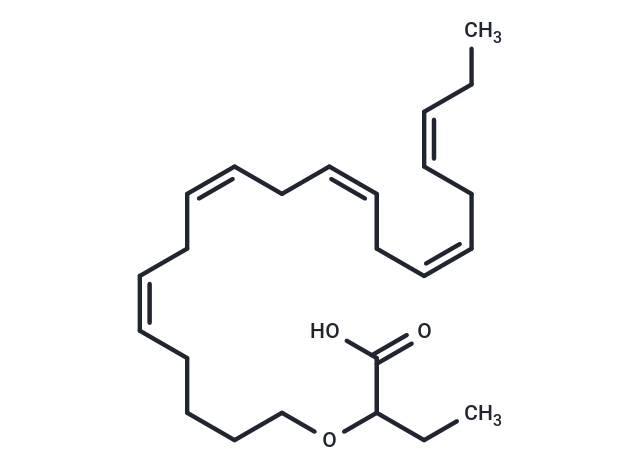
Icosabutate
CAS No. 1253909-57-7
Icosabutate ( —— )
产品货号. M33382 CAS No. 1253909-57-7
Icosabutate 是一种具有口服活性的 ω-3多不饱和脂肪酸,是二十碳五烯酸的衍生物 (EPA derivative)。Icosabutate 克服了未经修饰的 EPA 对肝脏靶向的缺点,可改善了胰岛素敏感性,肝炎和纤维化。Icosabutate 具有良好的耐受性,可有效降低持续性高甘油三酯血症中的非高密度脂蛋白胆固醇 (non-HDL-C) 水平。
纯度: >98% (HPLC)
 COA
COA
 Datasheet
Datasheet
 HNMR
HNMR
 HPLC
HPLC
 MSDS
MSDS
 Handing Instructions
Handing Instructions
| 规格 | 价格/人民币 | 库存 | 数量 |
| 2MG | ¥1150 | 有现货 |


|
| 5MG | ¥1591 | 有现货 |


|
| 10MG | ¥2398 | 有现货 |


|
| 25MG | ¥4841 | 有现货 |


|
| 50MG | ¥7765 | 有现货 |


|
| 100MG | ¥12470 | 有现货 |


|
| 200MG | ¥16754 | 有现货 |


|
| 500MG | 获取报价 | 有现货 |


|
| 1G | 获取报价 | 有现货 |


|
生物学信息
-
产品名称Icosabutate
-
注意事项本公司产品仅用于科研实验,不得用于人体或动物的临床与诊断
-
产品简述Icosabutate 是一种具有口服活性的 ω-3多不饱和脂肪酸,是二十碳五烯酸的衍生物 (EPA derivative)。Icosabutate 克服了未经修饰的 EPA 对肝脏靶向的缺点,可改善了胰岛素敏感性,肝炎和纤维化。Icosabutate 具有良好的耐受性,可有效降低持续性高甘油三酯血症中的非高密度脂蛋白胆固醇 (non-HDL-C) 水平。
-
产品描述Icosabutate, an orally active ω-3 polyunsaturated fatty acid, is an aeicosapentaenoic acid (EPA) derivative. Icosabutate overcomes the drawbacks of unmodified EPA for liver targeting and improves insulin sensitivity, hepatic inflammation and fibrosis. Icosabutate is well tolerated, and efficacious in lowering non-high-density lipoprotein cholesterol (non-HDL-C) levels in persistent?hypertriglyceridemia?.
-
体外实验——
-
体内实验Icosabutate (oral gavage; 100 mg/kg; once) accounts for the much higher flow rate of portal vein plasma (522?mL/h) versus mesenteric lymph (0.5?mL/h), that data demonstate thaticosabutate is almost entirely taken up through the portal vein (>99%) with only a small fraction of icosabutate being absorbed through the lymphatic pathway in 8‐week old male Wistar rats.Icosabutate ([14‐C]‐icosabutate; oral gavage; 100 mg/kg; once) shows that peak concentrations of radioactivity in most tissues at 4‐8 hours after the dose (except the gastrointestinal tract) with highest concentrations in the liver and kidney, most other tissues contain levels of radioactivity below that in plasma in male albino Wistar rats.Icosabutate (diet administration; 135?mg/kg/day; 5?weeks) markedly improved glucose tolerance after an oral glucose load, significantly reduces AUC (0‐120 minutes) by 60% without affecting body weight, decrease plasma alanine aminotransferase (ALT) levels improves glucose metabolism by a significant decrease in blood glucose, blood hemoglobin A1c, plasma insulin, and HOMA‐IR (-50%, -47%, -76% and -87%, respectively) in mice. =Icosabutate (oral adminstration; 112?mg/kg/day; 20 weeks) prevents microvesicular steatosis (-35%) and hepatocellular hypertrophy (-82%), but not macrovesicular steatosis. After 20 weeks of treatment, despite comparable decreases in hepatic inflammatory cell aggregates, only icosabutate reduced hepatic collagen content.Animal Model:6‐8‐week‐old male?ob/ob?mice Dosage:135?mg/kg Administration:135?mg/kg/day through diet administration; 5?weeks Result:Improved glucose metabolism and insulin resistance.Animal Model:8‐15‐week‐old male APOE*3Leiden. CETP mice fed a high‐fat and high cholesterol diet Dosage:112?mg/kg/day Administration:Oral gavage; 20 weeks Result:Improved microvesicular steatosis, hepatic inflammation, and fibrosis.
-
同义词——
-
通路Others
-
靶点Other Targets
-
受体Others
-
研究领域——
-
适应症——
化学信息
-
CAS Number1253909-57-7
-
分子量374.56
-
分子式C24H38O3
-
纯度>98% (HPLC)
-
溶解度In Vitro:?DMSO 中的溶解度 : 100 mg/mL (266.98 mM; 超声助溶 )
-
SMILESCC\C=C/C\C=C/C\C=C/C\C=C/C\C=C/CCCCOC(CC)C(O)=O
-
化学全称——
运输与储存
-
储存条件(-20℃)
-
运输条件With Ice Pack
-
稳定性≥ 2 years
参考文献
1. van den Hoek AM, et al. Icosabutate?Exerts?Beneficial?Effects?Upon?Insulin?Sensitivity,?Hepatic?Inflammation,?Lipotoxicity, and?Fibrosis?in?Mice.Hepatol Commun.?2019 Dec 24;4(2):193-207.?



 021-51111890
021-51111890 购物车()
购物车()
 sales@molnova.cn
sales@molnova.cn











