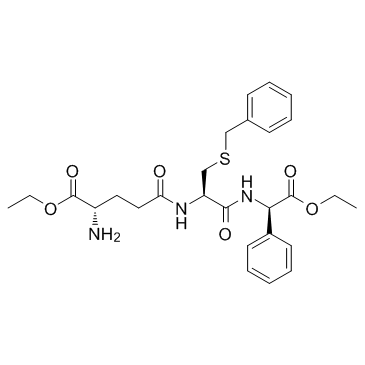
Ezatiostat
CAS No. 168682-53-9
Ezatiostat ( TER199(free base) | TLK199 )
产品货号. M20227 CAS No. 168682-53-9
Ezatiostat 是谷胱甘肽的三肽类似物,可以选择性抑制 GSTP1-1 催化活性。
纯度: >98% (HPLC)
 COA
COA
 Datasheet
Datasheet
 HNMR
HNMR
 HPLC
HPLC
 MSDS
MSDS
 Handing Instructions
Handing Instructions
| 规格 | 价格/人民币 | 库存 | 数量 |
| 5MG | ¥527 | 有现货 |


|
| 10MG | ¥915 | 有现货 |


|
| 25MG | ¥1661 | 有现货 |


|
| 50MG | ¥2770 | 有现货 |


|
| 100MG | ¥4722 | 有现货 |


|
| 200MG | 获取报价 | 有现货 |


|
| 500MG | 获取报价 | 有现货 |


|
| 1G | 获取报价 | 有现货 |


|
生物学信息
-
产品名称Ezatiostat
-
注意事项本公司产品仅用于科研实验,不得用于人体或动物的临床与诊断
-
产品简述Ezatiostat 是谷胱甘肽的三肽类似物,可以选择性抑制 GSTP1-1 催化活性。
-
产品描述Ezatiostat is a tripeptide analog of glutathione that can selectively inhibit GSTP1-1 catalytic activity.
-
体外实验Ezatiostat causes dissociation of the enzyme from the jun-N-terminal kinase/c-Jun (JNK/JUN) complex, leading to JNK activation by phosphorylation. The therapeutic action of ezatiostat appears to include both proliferation of normal myeloid progenitors as well as apoptosis of the malignant clone.Selection of a resistant clone of an HL60 tumor cell line through chronic exposure to Ezatiostat (TLK199) results in cells with elevated activities of c-Jun NH2 terminal kinase (JNK1) and ERK1/ERK2, and allowes the cells to proliferate under stress conditions that induced high levels of apoptosis in the wild type cells.
-
体内实验Administration of Ezatiostat (TLK199), stimulates both lymphocyte production and bone marrow progenitor (colony-forming unit-granulocyte macrophage) proliferation, but only in glutathione S-transferase P1-1 (GSTP1+/+) and not in GSTP1-/- animals.
-
同义词TER199(free base) | TLK199
-
通路Cytoskeleton/Cell Adhesion Molecules
-
靶点GST
-
受体GSTP1-1
-
研究领域Inflammation/Immunology
-
适应症Myelodysplastic syndromes; Neutropenia
化学信息
-
CAS Number168682-53-9
-
分子量529.65
-
分子式C27H35N3O6S
-
纯度>98% (HPLC)
-
溶解度DMSO: >50 mg/mL (94.4 mM);Ethanol: 20 mg/mL (37.76);Water: Insoluble
-
SMILESCCOC(=O)[C@@H](N)CCC(=O)N[C@@H](CSCc1ccccc1)C(=O)N[C@@H](C(=O)OCC)c1ccccc1
-
化学全称——
运输与储存
-
储存条件(-20℃)
-
运输条件With Ice Pack
-
稳定性≥ 2 years
参考文献
1.Raza A et al. Phase 1 dose-ranging study of ezatiostat hydrochloride in combination with lenalidomide in patients with non-deletion (5q) low to intermediate-1 risk myelodysplastic syndrome (MDS). J Hematol Oncol. 2012 Apr 30;5:18.



 021-51111890
021-51111890 购物车(0)
购物车(0)
 sales@molnova.cn
sales@molnova.cn











