Delanzomib
CAS No. 847499-27-8
Delanzomib ( CEP-18770 | CEP18770 | CEP 18770 )
产品货号. M16150 CAS No. 847499-27-8
一种有效的口服活性蛋白酶体抑制剂,针对胰凝乳蛋白酶样蛋白酶体活性的 IC50 为 3.8 nM。
纯度: >98% (HPLC)
 COA
COA
 Datasheet
Datasheet
 HNMR
HNMR
 HPLC
HPLC
 MSDS
MSDS
 Handing Instructions
Handing Instructions
| 规格 | 价格/人民币 | 库存 | 数量 |
| 2MG | ¥486 | 有现货 |


|
| 5MG | ¥867 | 有现货 |


|
| 10MG | ¥1644 | 有现货 |


|
| 25MG | ¥3216 | 有现货 |


|
| 50MG | ¥4836 | 有现货 |


|
| 100MG | ¥6691 | 有现货 |


|
| 200MG | 获取报价 | 有现货 |


|
| 500MG | 获取报价 | 有现货 |


|
| 1G | 获取报价 | 有现货 |


|
生物学信息
-
产品名称Delanzomib
-
注意事项本公司产品仅用于科研实验,不得用于人体或动物的临床与诊断
-
产品简述一种有效的口服活性蛋白酶体抑制剂,针对胰凝乳蛋白酶样蛋白酶体活性的 IC50 为 3.8 nM。
-
产品描述A potent, orally active proteasome inhibitor with IC50 of 3.8 nM against chymotrypsin-like proteasome activity; demonstrates marginal inhibition of the tryptic and peptidyl glutamyl activities of the proteosome; down-modulates the NF-kappaB activity and the expression of several NF-kappaB downstream effectors, induces apoptotic cell death in MM cells; demonstrates tumor regression in MM xenografts with favorable tumor selectivity profile.Blood Cancer Phase 1 Discontinued(In Vitro):Delanzomib (CEP-18770; 20 nM; 12-24 hours) treatment results in a progressive appearance of cleaved caspases-3, -7, and -9 between 12 and 24 hours’exposure in the human MM cell lines, RPMI-8226, and U266.Delanzomib (CEP-18770; 5-40 nM; 4-24 hours) treatment induces an accumulation of ubiquitinated proteins over 4 to 8 hours.Delanzomib (CEP-18770) inhibits endothelial cell survival, vasculogenesis, and osteoclastogenesis in vitro; and displays a favorable cytotoxicity profile toward normal cells.(In Vivo):Delanzomib (CEP-18770; 7.8-13 mg/kg; oral administration; twice a week; for 4 weeks) treatment results in a more sustained pharmacodynamic inhibition of proteasome activity in tumors relative to normal tissues, complete tumor regression of multiple myeloma (MM) xenografts and improves overall median survival in a systemic model of human MM.
-
体外实验Delanzomib (CEP-18770; 20 nM; 12-24 hours) treatment results in a progressive appearance of cleaved caspases-3, -7, and -9 between 12 and 24 hours’exposure in the human MM cell lines, RPMI-8226, and U266. Delanzomib (CEP-18770; 5-40 nM; 4-24 hours) treatment induces an accumulation of ubiquitinated proteins over 4 to 8 hours.Delanzomib (CEP-18770) inhibits endothelial cell survival, vasculogenesis, and osteoclastogenesis in vitro; and displays a favorable cytotoxicity profile toward normal cells. Apoptosis Analysis Cell Line:RPMI-8226, U266, and K562 cells Concentration:20 nM Incubation Time:12 hours, 24 hours Result:Resulted in a progressive appearance of cleaved caspases-3, -7, and -9 between 12 and 24 hours’exposure in the human MM cell lines.Western Blot Analysis Cell Line:RPMI-8226, U266, and K562 cells Concentration:5 nM, 10 nM, 20 nM, 40 nM Incubation Time:4 hours, 8 hours, 12 hours, 24 hours Result:Induced an accumulation of ubiquitinated proteins over 4 to 8 hours.
-
体内实验Delanzomib (CEP-18770; 7.8-13 mg/kg; oral administration; twice a week; for 4 weeks) treatment results in a more sustained pharmacodynamic inhibition of proteasome activity in tumors relative to normal tissues, complete tumor regression of multiple myeloma (MM) xenografts and improves overall median survival in a systemic model of human MM. Animal Model:SCID mice injected with RPMI 8226 cells Dosage:7.8 mg/kg, 10 mg/kg, 13 mg/kg Administration:Oral administration; twice a week; for 4 weeks Result:Resulted in a more sustained pharmacodynamic inhibition of proteasome activity in tumors relative to normal tissues.
-
同义词CEP-18770 | CEP18770 | CEP 18770
-
通路Proteasome/Ubiquitin
-
靶点Proteasome
-
受体Chymotrypsin-likeproteasome
-
研究领域Cancer
-
适应症Blood cancer
化学信息
-
CAS Number847499-27-8
-
分子量413.2751
-
分子式C21H28BN3O5
-
纯度>98% (HPLC)
-
溶解度10 mM in DMSO
-
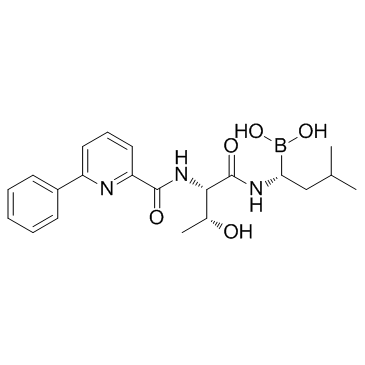
SMILESCC(C)C[C@@H](B(O)O)NC([C@@H](NC(C1=NC(C2=CC=CC=C2)=CC=C1)=O)[C@H](O)C)=O
-
化学全称Boronic acid, B-[(1R)-1-[[(2S,3R)-3-hydroxy-1-oxo-2-[[(6-phenyl-2-pyridinyl)carbonyl]amino]butyl]amino]-3-methylbutyl]-
运输与储存
-
储存条件(-20℃)
-
运输条件With Ice Pack
-
稳定性≥ 2 years
参考文献
1. Piva R, et al. Blood. 2008 Mar 1;111(5):2765-75.
2. Sanchez E, et al. Br J Haematol. 2010 Feb;148(4):569-81.
3. Seavey MM, et al. Int Immunopharmacol. 2012 Jan;12(1):257-70.
4. Berkers CR, et al. Mol Pharm. 2012 May 7;9(5):1126-35.



 021-51111890
021-51111890 购物车(0)
购物车(0)
 sales@molnova.cn
sales@molnova.cn











